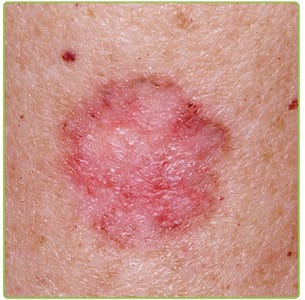
In late July, 2015, the FDA approved a new skin cancer drug for the treatment of basal cell carcinoma skin cancer. The drug, which will be marketed under the name Odomzo (generic name sonidegib) and will be available to adults in 200mg doses, is produced by Swiss drug manufacturer Novartis Pharmaceuticals.
Odomzo will be used to treat locally advanced basal cell carcinomas in patients where the disease has returned after the original tumor was surgically removed. The drug will also be an option for patients who are not candidates for surgery or radiation treatment.